paper archives
Stay hungry, stay foolish. You are as good as your last paper.
Graphitic carbon nitride: Effects of various precursors on the structural, morphological and electrochemical sensing properties
- Hui Lin Lee, Zdeněk Sofer, Vlastimil Mazánek, Jan Luxa, Chun Kiang Chua, Martin Pumera*

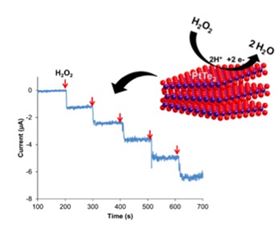
Graphitic carbon nitride (g-C3N4) has been discovered very long ago, in the 1830s. g-C3N4, an analogue of graphene, has been of great interest due to the strong electron donor nature of nitrogen present in g-C3N4, which is absent in graphene. Though studies have shown that g-C3N4 can be used as an electrochemical sensing platform for electrochemical study of H2O2, glucose, mercuric ions, nitrobenzene and reduced nicotinamide adenine dinucleotide (NADH), few studies have been conducted to investigate the electrochemical behaviours of g-C3N4 produced by various precursors (bulk condensation pyrolysis of nitrogen-rich precursors): trithiocyanuric acid (C3N4-TC), triazinetrihydrazine (C3N4-TH), cyanuric acid (C3N4-CA), cyanuric chloride (C3N4-CC), dicyandiamide (C3N4-DD) and melamine (C3N4-ME) on important biomarkers: ascorbic acid, dopamine, uric acid and adenine and to observe any enhanced performance over glassy carbon (GC). In this work, the performance of g-C3N4 materials on the electrochemical sensing of biomarkers is analysed with cyclic voltammetry and differential pulse voltammetry techniques. Among the g-C3N4 materials tested, C3N4-TC and C3N4-CA surfaces exhibited lower over-potentials for the electrochemical sensing of the biomarkers as compared to GC. In addition, these two surfaces showed great sensitivity and good linearity for the biomarkers tested. This work provides a good understanding of the electrochemical properties of g-C-3 N-4 materials synthesized from various precursors as well as the effectiveness of the g-C-3 N-4 materials as electrochemical sensing platform. (C) 2016 Elsevier Ltd. All rights reserved.